
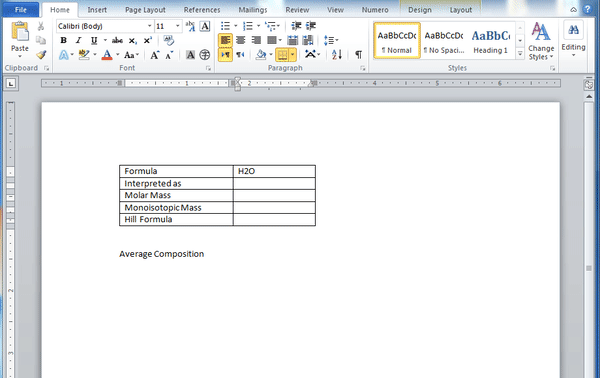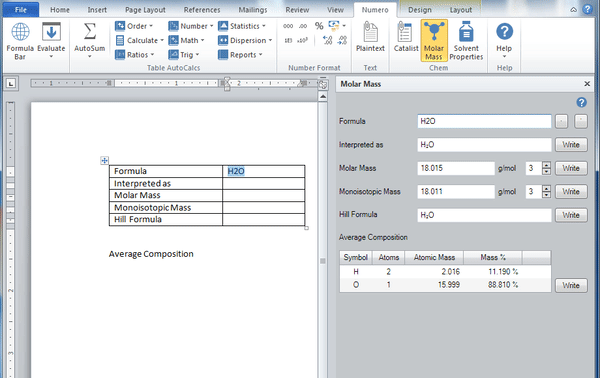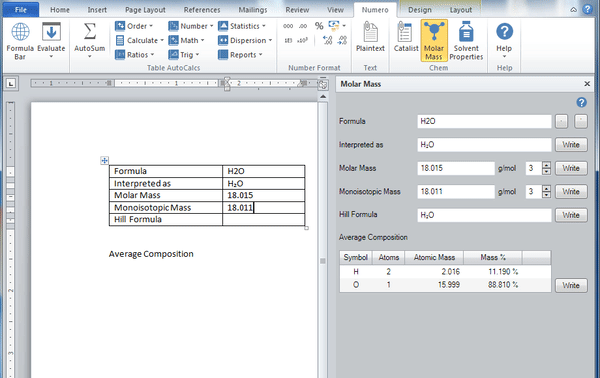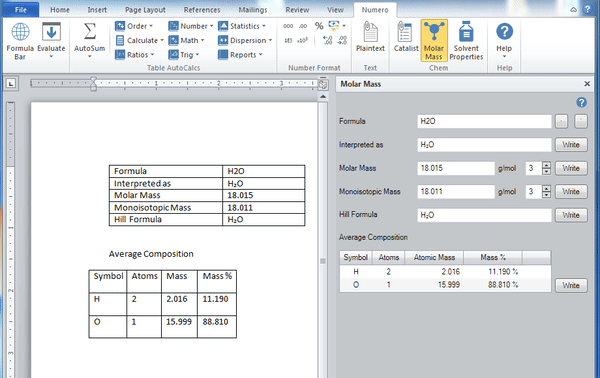
Molar mass calculator also displays common compound name, Hill formula, elemental composition, mass percent composition, atomic percent compositions and allows to convert from weight to number of moles.
#Cuso4 molar mass how to#
Other heavy metals should be collected separately. Os exemplos de clculos de massa molar: NaCl, Ca(OH)2, K4Fe(CN)6, CuSO45H2O, water, nitric acid, potassium permanganate, ethanol, fructose. Explanation of how to find the molar mass of CuSO4: Copper (II) sulfate.A few things to consider when finding the molar mass for CuSO4:- make sure you have t. Anhydrous copper sulfate is 39.81 copper and 60.19 sulfate by mass, and in its blue, hydrous form, it is 25. 5H2O Atomic mass of Cu + Atomic mass of S + (9 x atomic mass of O) + (10 x atomic mass of H) Hence, the molecular mass of CuSO4. to AbfallverzeichnisV - Waste Catalogue Ordinance, Appendix 3). Molar mass: 159.60 g/mol (anhydrous) 249.685 g/mol (pentahydrate) Appearance gray. The molecular mass of a compound is the sum of the atomic masses of all the constituent molecules. In this context, heavy metal means any compound of antimony, arsenic, cadmium, chromium(VI), copper, lead, nickel and tin, as well as these subtances in metallic form, if they are classified as hazardous (acc. Neither Raney nickel itself nor any filter residues should be allowed to dry out, otherwise they will spontaneously ignite in air. Stir Raney nickel (also: Urushibara nickel) in the form of an aqueous suspension into hydrochloric acid (Cat. P391: Collect spillage.ġ5 Heavy metal-containing solutions and solids: container E. cuso4 + 2 h2o > cu(oh)2 + h2 so4 As we can see, copper hydroxide and sulphuric acid is generated in this chemical reaction.Copper hydroxide is a weak base whereas sulphuric acid is a very strong acid which results into increase in acidity of the solution. Remove contact lenses, if present and easy to do. What is the mass of 0.75 moles of CuSO4 If you work out the relative formula mass, which is one mole, then you find 0.75 of that. NaCl CaC4H4O6 MgCrO4 Al2(SO4)3 K3PO4 ZnCl2 COMPOUND FORMULA MASS CuSO4. The anhydrous copper sulfate (CuSO4) has a molar mass of 159,62. P305 + P351 + P338: IF IN EYES: Rinse cautiously with water for several minutes. Solutions to the Molar Mass Practice Worksheet: Important note to students: All. P301 + P312: IF SWALLOWED: Call a POISON CENTER/doctor if you feel unwell. P280: Wear eye protection/ face protection.
#Cuso4 molar mass skin#
P264: Wash skin thoroughly after handling. H410: Very toxic to aquatic life with long lasting effects. Atomic mass of one Copper atom 63.5 amu Atomic mass of one Sulpur atom 32 amu Atomic mass of one Oxygen atom 16 amu Atomic mass of four Oxygen atoms 4×16 amu 64 amu. CX2185 CX2185-1 CX2185-5 CX2185-7 CX2185-20Ĭopper monosulfate pentahydrate, Copper vitriol pentahydrate Step 2: Atomic mass of each atom in CuSO 4: Copper sulfate ( CuSO 4) has one Copper ( Cu) atom, one Sulphur S atom, and four Oxygen O atoms.


 0 kommentar(er)
0 kommentar(er)
